Laws of Thermodynamics
Thermodynamics is the study of how heat moves through systems and interacts with matter. Like other branches of physics, thermodynamics is governed by laws, which offer profound implications, such as explaining why time can not move backward.
Key terms
To fully understand the laws of thermodynamics, we first must define some key terms:
- Entropy: A measure of the disorder in a system. For instance, a system of particles with higher kinetic energy has greater entropy than those with lower kinetic energy in the same volume.
- Thermodynamical equilibrium: A state in which a system’s properties remain constant over time, with no energy exchange between systems. At equilibrium, entropy is at its maximum.
- Internal energy U: total energy within a system, including kinetic and potential energy
- Work W: the work done on the system
- Heat Q: heat added to a system
The zeroth law of thermodynamics
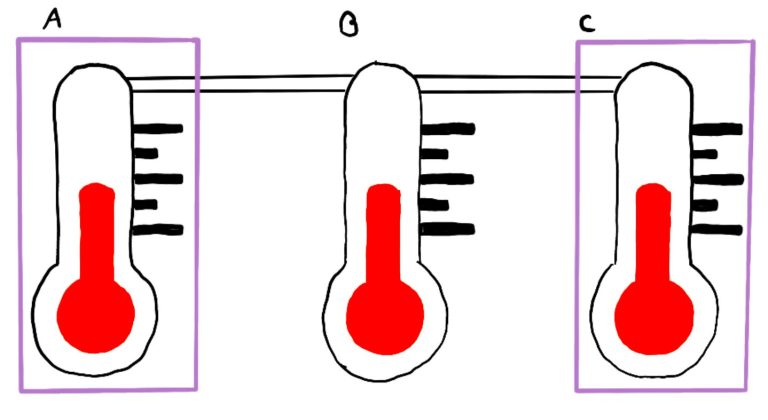
If system A and B are in thermodynamical equilibrium, and B is in equilibrium with C, then A is in equilibrium with C.
This law is about the thermodynamical equilibrium of various systems and provides the basis for temperature measurement.
Two systems are in equilibrium when no observable changes occur within them.
Sub-systems like A, B, and C interact via either mechanical contact or thermal contact. Mechanical contact means that a moveable wall separates the systems, allowing pressure to equalize. Thermal contact means that a thermally conductive (diathermal) wall permits heat to flow between systems until their temperatures equalize.
The zeroth law was basic understanding and considered to be true but needed to be formally stated. Ralph Fowler called it the zeroth law because the others were already numbered, and the zeroth law is the foundation for the others.
The first law of thermodynamics
The internal energy of an isolated system is conserved under any thermodynamical change.
Conclusively, in a non- isolated system, any change in the internal energy equals the difference between the heat added to the system and the work done by the system on its surrounding: U=Q-W
This law essentially reflects the principle of energy conservation, showing that energy con neither be created nor destroyed, only transformed or transferred.
The second law of thermodynamics
The entropy of an isolated system always increases over time.
Heat always flows from a warmer region to a cooler one, never the reverse, unless external work is applied.
This law explains why all systems evolve toward thermodynamical equilibrium, where entropy is maximized, and no energy is available to do any useful work.
Additionally, it introduces the concept of the „arrow of time “, as processes with increasing entropy are irreversible. This is why the beginning of the universe lies in the past and not the future.
The third law of thermodynamics
The entropy of an isolated system at thermodynamic equilibrium approaches a constant value when its temperature approaches absolute zero.
Absolute zero, or 0 Kelvin (-273.15C), is the lowest possible temperature in the universe, where particles no longer possess kinetic energy and motion ceases completely. However, cooling down a system to absolute zero is practically impossible, as the steps required to remove energy gorw infinitely larger as the temperature decreases.
Absolute zero exists only as a theoretical construct.
©Copyright. All rights reserved.
Wir benötigen Ihre Zustimmung zum Laden der Übersetzungen
Wir nutzen einen Drittanbieter-Service, um den Inhalt der Website zu übersetzen, der möglicherweise Daten über Ihre Aktivitäten sammelt. Bitte überprüfen Sie die Details in der Datenschutzerklärung und akzeptieren Sie den Dienst, um die Übersetzungen zu sehen.