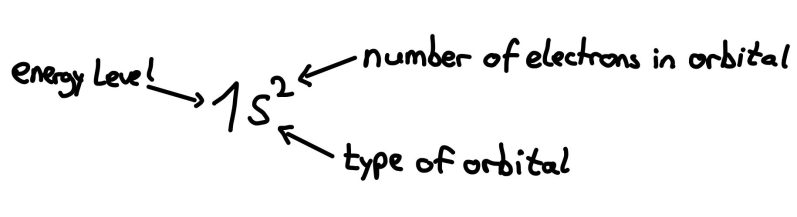
Orbitals
An orbital is a three-dimensional region around an atom where an electron is most likely to be found.
Uncertainty Principle
In quantum mechanics, we cannot precisely determine the exact state of a particle. According to Heisenberg’s Uncertainty Principle, we cannot know both the position and momentum of an electron at the same time. The product of the uncertainty in position and the uncertainty of momentum must be greater than or equal to Planck’s constant (h).
This means that the exact position and velocity of an electron cannot be known simultaneously.
Quantum tunneling exploits this uncertainty, allowing particles to pass through energy barriers that would be insurmountable in classical physics. Click here to learn more.
Probability and interpretation of orbitals
Orbitals do not represent paths of electrons but rather probability distributions. They are an assumption of where the electron might be. The likelihood of finding the electron within a given orbitals is approximately 90%.
As the number of electrons in an atom increases, more orbitals are needed to describe the atom‘s electron distribution. These orbitals vary in energy level, shape and orientation.
Key properties of orbitals
- Each orbital can hold a maximum of two electrons
- Electrons in the same orbital must have opposite spins, according to the Pauli Exclusion Principle.
- Orbitals differ in shape and are classified as s, p, d, and f orbitals (represented by the angular momentum quantum number
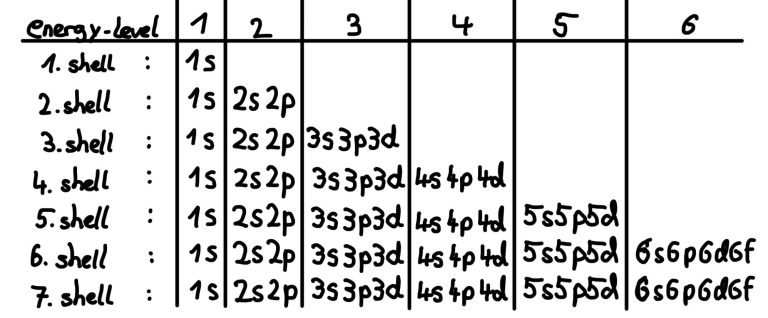
- Orbitals have different energy levels, similar to electron shells in the shell model (represented by the principle quantum number)
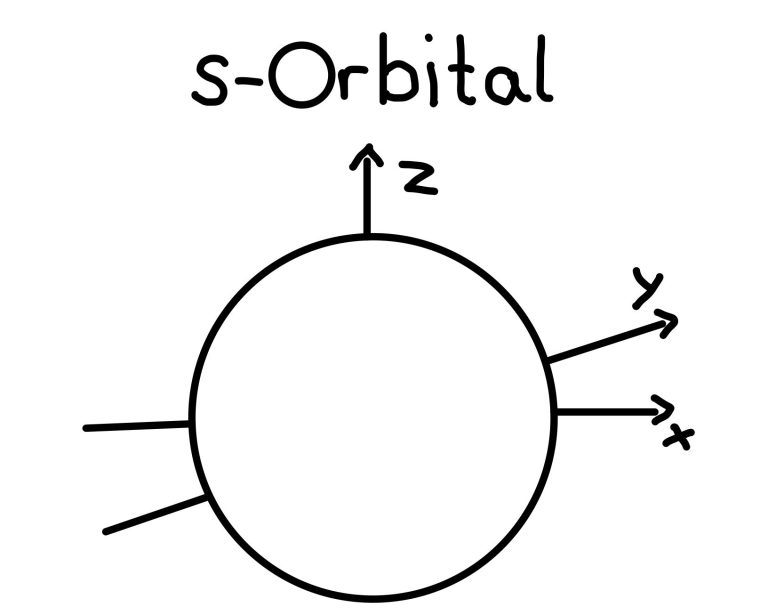
S-Orbitals
The s-orbital is the simples type of orbitals. It is spherically symmetrical in shape.
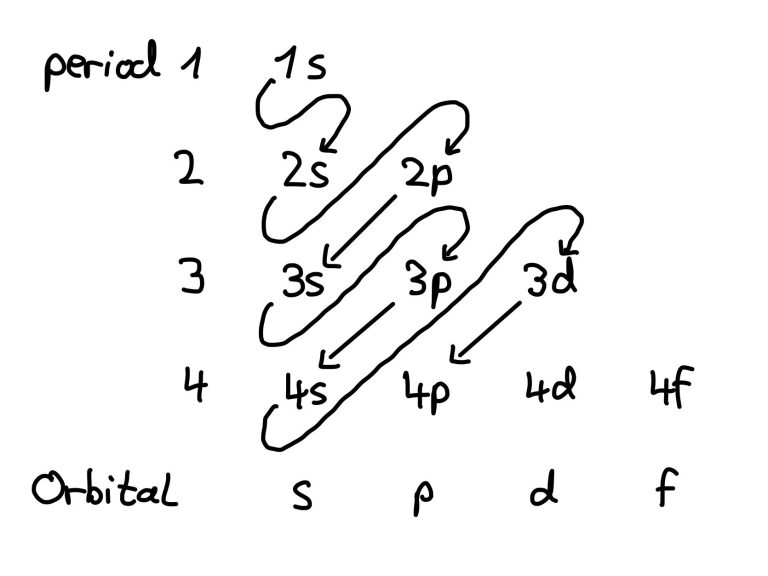
Each energy level contains one s-orbitals (eg. 1s, 2s, 3s...). Each period in the periodic table represents one energy level.
For instance, the first-period elements, hydrogen (H) and helium (He), have their electrons in the 1s orbital.
Lithium (Li), in the second period, distributes its three electrons in the 1s and 2s orbital.
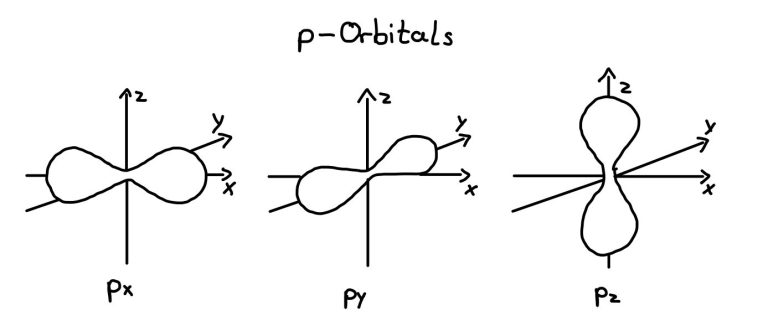
P-Orbitals
P-orbitals exist from the second energy level onward (starting with boron, Z=5)
Each energy level from n=2 onward has three p-orbitals, orientated along the x, y, and z axed. They are labelled px-orbital, py-orbital, and pz-orbital and each can hold two electrons, meaning that all all three p-orbitals can accommodate six electrons.
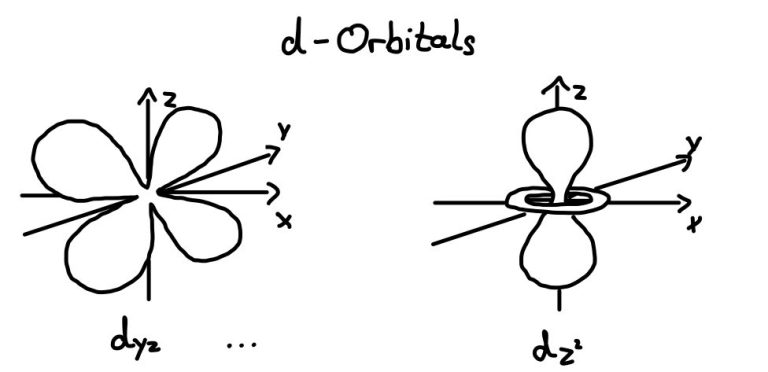
D-orbital
There is no 1d or 2d orbitals; d-orbitals appear for electrons at higher energy levels, from n=3 onward. A total of five d-orbitals exist per energy level, starting from 3d.
Notably, the 4s orbital fills before the 3d orbital, despite 3d belonging to the third shell. This happened because the 4s orbital is lower in energy than the 3d orbitals.
F-orbitals
F-orbitals appear from the fourth energy level (4f) and contain seven orbitals, allowing up to 14 electrons.
They have complex shapes , influencing chemical properties like magnetism and color and are filled in lanthanides (4f) and actinides (5f).
Example
Let‘s look at an example of the orbitals occupancy rules: the carbon atom. It has six electrons, requiring at least three orbitals for their distribution.
- The 1s orbital is filled with two electrons.
- Then the 2s orbitals is also filled with two electrons.
- The 2p orbitals (px, py, pz) receive the remaining two electrons.
Since the p orbitals are energetically equal, the two electrons are distributed to two of the three p-orbitals.
Electron configuration
For the electron configuration, you first write down the occupied orbitals in energetic order and insert the number of electrons in each orbital into the exponent.
Carbon‘s electron configuration is:
1s^2 2s^2 2p^2
Orbitals with the same energy (px, py, pz) are grouped together.
Iron:
Iron has 26 electrons. The electron configuration is:
1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6
Orbital Occupation Rules
- Electrons fill orbitals in order of increasing energy (Aufbau principle)
- Each orbital can hold a maximum of two electrons with opposite spins (Pauli Exclusion Principle)
- Orbitals of equal energy are filled singly before pairing begins (Hund‘s rule)
©Copyright. All rights reserved.
Wir benötigen Ihre Zustimmung zum Laden der Übersetzungen
Wir nutzen einen Drittanbieter-Service, um den Inhalt der Website zu übersetzen, der möglicherweise Daten über Ihre Aktivitäten sammelt. Bitte überprüfen Sie die Details in der Datenschutzerklärung und akzeptieren Sie den Dienst, um die Übersetzungen zu sehen.