Electromagnetic Waves
Electromagnetic waves are an essential part of our lives, influencing everything from how we see to how we understand the universe.
For instance, the reading a book involves electromagnetic waves being reflected from its surface and entering your eyes.
Frequency and wavelength
Whether you see green, blue or any other color depends on the frequency of the wave. Frequency refers to the number of wave crests passing a point in a given time, and it determines the energy of the wave.
Waves have different wavelengths. The wavelength is inversely proportional to the frequency, which means that higher- frequency waves have shorter wavelengths. The relationship between wavelength (λ) and frequency (f) is f=c/λ, where c is the speed of light, approximately 299792458 m/s.
Photons
The wave-particle-dualism is a principle from quantum mechanics that describes light as both an electromagnetic wave and a particle called the photon. Photons are packets of energy, and their energy can be calculated using E=hf or E=hc/λ, the energy of an electromagnetic wave.
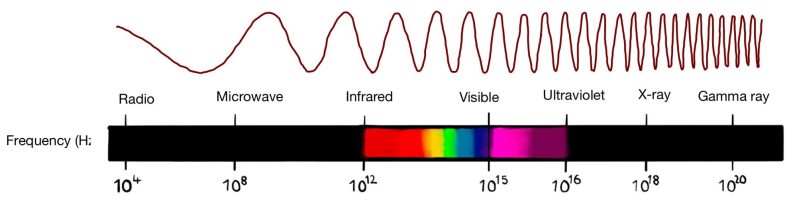
Electomagnetic spectrum
Humans can perceive only electromagnetic waves with wavelength from 400nm-700nm which is roughly the seize of a cell. However, there is a wide range of wavelengths:
Animals and the spectrum
Interestingly, many animals can see a different part of the electromagnetic spectrum due to different receptors in their eyes. Humans miss out of most of the waves that they are surround by.
However, many animals, such as insects, birds and even reindeers can see ultraviolet waves which are invisible for us. This ability gives them a perception of the world that vastly differs from our own, revealing patterns and details hidden to the human eye.
Application of the spectrum
The electromagnetic spectrum spans a wide range of wavelengths and frequencies, from radio waves with the longest wavelengths to gamma rays with the shortest. These waves gave countless applications in science and technology.
For instance, they are used to analyze the structure of materials based on the fact that different atoms and molecules absorb and reflect different wavelengths. However, the small size of elementary particles poses a limitation.
Even gamma rays, which have the shortest wavelengths, are too large to interact with particles like quarks or electrons effectively, preventing us from using electromagnetic waves to directly image these particles.
Fraunhofer spectrum
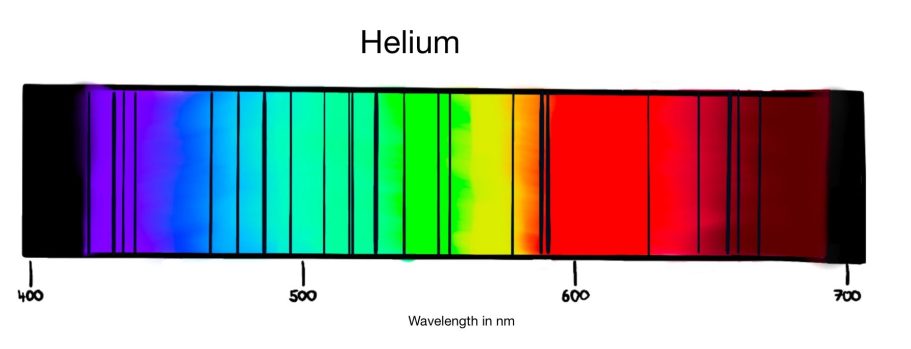
One fascinating application of this spectrum is the study of Fraunhofer lines- dark lines observed in the spectrum of sunlight. These lines were discovered by physicist Joseph von Fraunhofer when he recorded the spectrum of the sun and observed thin black lines in this spectrum.
They represent wavelengths of light absorbed by elements in the sun's atmosphere.
Exciting electrons
The sun emits light, which passes through its atmosphere before reaching us. The atmosphere contains elements like helium, whose electrons absorb specific wavelengths of light.
According to Bohrs atomic model, electrons orbit the nucleus in shells and can absorb energy to jump to a higher shell. The energy corresponds to a specific wavelength and frequency, described by E=hf.
Once excited, electrons quickly return to their original shell due to a basic law in physics which claims that an excited system always wants to return to the position of lowest energy as fast as possible. The electrons release the absorbed energy as light in a random direction which removes specific wavelengths from the sun's spectrum, creating dark lines unique to each element. For example, the one of helium:
By analyzing these lines scientists can determine the composition of the sun's atmosphere.
The origin of electromagnetic waves
Electromagnetic waves are created by moving charged particles, like electrons or protons.
A stationary particle generates an electric field around it, but when the particle moves, it also produces a magnetic field. These electric and magnetic fields are independent and interact to form an electromagnetic wave.
At a deeper level, particles like electrons possess a quantum property called spin, which can be thought of as a rotation of the particles around their axis. This rotation generates an electromagnetic field.
When the particles move, their fields oscillate and spread through space at the speed of light, creating electromagnetic waves.
In atoms, electrons behave as if connected to the nucleus by an elastic band. When displaced, these electron clouds vibrate, producing electromagnetic waves. These waves can travel vast distances and interact with other particles by disturbing their electromagnetic fields.
This is how sunlight heats the earth despite the vast distance between them.
©Copyright. All rights reserved.
Wir benötigen Ihre Zustimmung zum Laden der Übersetzungen
Wir nutzen einen Drittanbieter-Service, um den Inhalt der Website zu übersetzen, der möglicherweise Daten über Ihre Aktivitäten sammelt. Bitte überprüfen Sie die Details in der Datenschutzerklärung und akzeptieren Sie den Dienst, um die Übersetzungen zu sehen.