The Photoelectric effect
Albert Einstein received the Nobel Prize for his explanation of the photoelectric effect, a discovery that revolutionized modern physics.
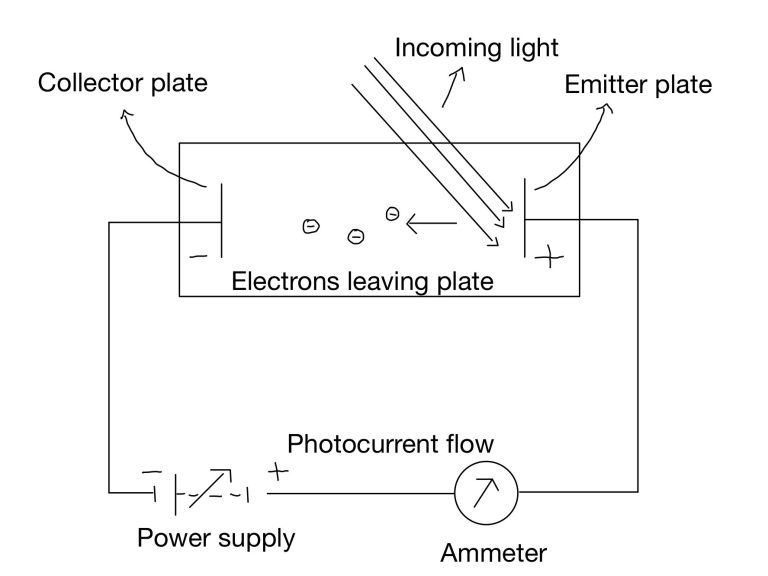
Experimental setup:
The experimental setup for observing the photoelectric effect consists of an emitter plate and a collector plate connected to a power supply, with and ammeter included to measure the current. When light shines on the emitter plate, electrons are ejected from its surface, travel through the vacuum, and reach the collector plate.
These electrons complete the circuit, and their flow generates a measurable current. The presence of this current is the hallmark of the photoelectric effect.
Observations:
- Photoelectric emission of electrons is emitted only if the frequency of the incident electromagnetic radiation exceeds a specific threshold, known as the Threshold frequency.
- The number of electrons emitted per second is proportional to the intensity of the light, provided the lights frequency is above the threshold frequency.
- Photoelectric emission occurs without delay as soon as the incident radiation strikes the emitter plate, regardless of the light's intensity.
Complications with classical mechanics:
In classical mechanics, light is treated as a wave and the energy of the wave is distributed evenly across the wavefront. This leads to the following contradictions:
- If light were spread out across the wavefront, an electron would require time to absorb enough energy to escape the metal surface, leading to a delay in emission. Yet, no such delay is observed.
- According to classical mechanics, any frequency of light should eventually be able to provide enough energy for electrons to escape, given high enough intensity. However, experiments show that of the frequency is below the threshold frequency, increasing intensity has no effect.
- Classical theory predicts that increasing intensity should increase the kinetic energy of emitted electrons. Yet, experiments reveal that kinetic energy depends solely on the light’s frequency, while intensity affects only the number of emitted electrons.
Einsteins explanation:
Einstein resolved these contradictions by introducing the concept of photons, discrete energy packets of light (wave-partice-duality). Each photon as energy E=hf, where f is the frequency of light and h is Plancks constant.
When light strikes the metal surface, an electron absorbs a single photon and gains its energy.
For an electron to escape the metal surface, the energy it absorbs must exceed the work function of the metal. The work function is the minimum energy required to liberate an electron.
The Threshold frequency is the minimum frequency required for a photons energy to equal the work function.
Increasing the intensity of the incident radiation on the plate increases the number of photons, leading to more emitted electrons (higher current) if the frequency exceeds the threshold frequency. However, the kinetic energy of the emitted electrons depends only on the frequency of the light. Each photon releases at most one electron in case it has enough energy.
Equation:
The left-hand side of the equation for the photoelectric effect represents the energy of the light striking the emitting plate. The photons of the light have the frequency f.
On the right-hand side is the total energy that the electron receives from the photon. This is the sum of the work function and the kinetic energy of the electron.
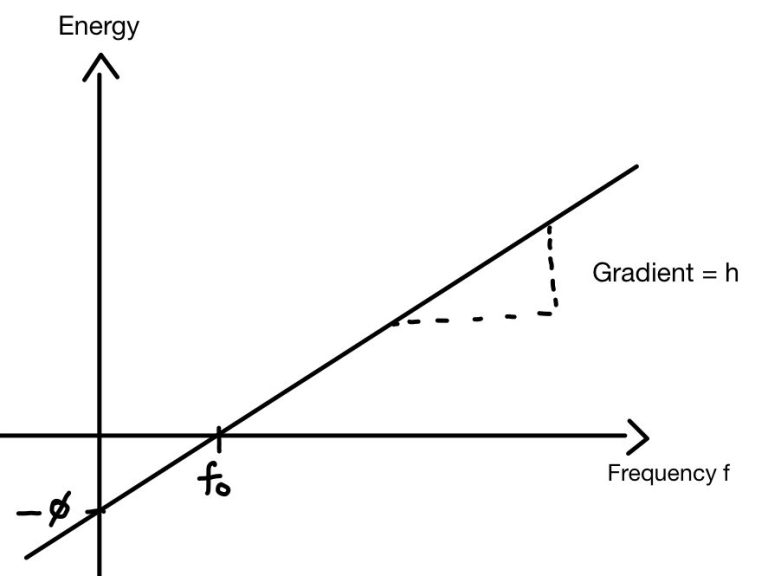
Graph:
Graphically, a plot of kinetic energy versus frequency reveals that no electrons are emitted with frequencies below the Threshold frequency (f₀), as photons lack the energy to overcome the work function.
With frequencies above f₀, kinetic energy of the electrons increases linearly with frequency. The gradient of the graph is Plank’s constant h and the y-intercept corresponds to the negative work function, the energy needed to free an electron from the surface.
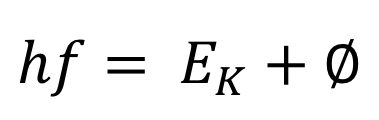
©Copyright. All rights reserved.
Wir benötigen Ihre Zustimmung zum Laden der Übersetzungen
Wir nutzen einen Drittanbieter-Service, um den Inhalt der Website zu übersetzen, der möglicherweise Daten über Ihre Aktivitäten sammelt. Bitte überprüfen Sie die Details in der Datenschutzerklärung und akzeptieren Sie den Dienst, um die Übersetzungen zu sehen.