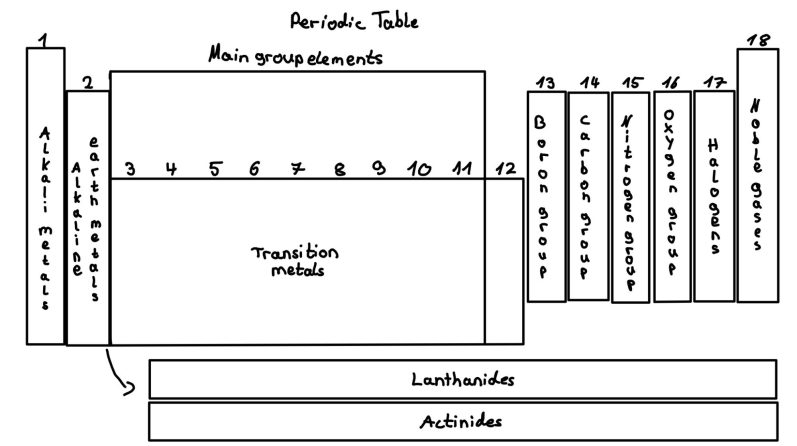
Periodic Table
The periodic table was invented by Dimitri Mendeleev in 1869. He aimed to arrange the known elements according to their properties. Despite missing elements at the time, he successfully predicted the properties of several yet-to-discover elements.
Key properties of the Periodic Table
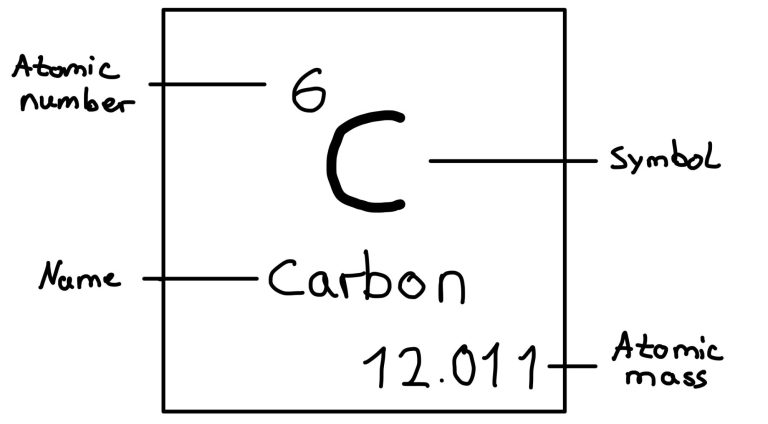
- Elements are characterized by their atomic number (Z), found in the box at the top left. Example: Carbon (C) -> Z=6
- Elements are arranged in rows (periods) and columns (groups)
- Elements within a group often have similar propertied, while elements in the same period have the same number of atomic orbitals (click here to learn more about orbitals)
Structure of an Element Box
Each element in the periodic table is represented in a box that contains:
- Atomic number (Z): number of protons in the nucleus
- Symbol: one- or two-letter abbreviation (e.g. C for carbon)
- Average atomic mass: weighted average of all isotopes of the element, e.g. chlorine has the chlorine-35 and chlorine-37 isotope
The periodic table consists of 118 elements with atomic number ranging from 1 (hydrogen) to 118 (oganesson). Elements with high atomic number (Z<92, transuranium elements) are unstable and only exist in laboratories as they decay rapidly.
Periods of the Periodic Table
Periods are the horizontal rows in the periodic table. There are seven periods, each corresponding to the energy level.
In the first three periods, the electrons of the element are distributed first in the ns orbital and then in the np orbital (n=principle quantum number)
- First period (n=1): electrons fill the 1s orbital (H, He)
- Second period (n=2): electrons fill the 2s and 2p orbitals (Li -> Ne)
- Third period (n=3): electrons fill the 3s and 3p orbitals (Na -> Ar)
Transition Elements and d-Orbitals
Starting from the fourth period, when n=4, d-orbitals begin to play a role.
In the fourth period (n=4), electrons fill the 4s orbital first, then 3d (Sc -> Zn) and then the 4p orbitals. The 4s orbital is lower in energy than the 3d orbital, so it fills first.
In the fifth period, electrons fill 5s, then 4d (Y -> Cd) and then 5p.
Lanthanides and Actinides (f-Orbitals)
Below the main table, the lanthanides (4f orbitals) and actinides (5f orbitals) form two separate rows.
Lanthanides with principle quantum number n=6 additionally occupy the 4f orbital. They are rare earth metals and highly reactive.
Actinides with principle quantum number n=7 additionally occupy the 5f orbital. They are highly radioactive elements, including uranium (U).
Groups in the periodic table
The periodic has 18 columns (groups). Groups 1-2 and 13-18 are the main groups, while 2-12 are transition metals.
The main groups include:
1: Alkali metals: Li, Na, K, Rb, Cs, very reactive, 1 valence electron
2: Alkaline earth metals: Be, Mg, Ca, Sr, Ba, reactive, 2 valence electrons
13: Boron group: B, Al, Ga, In, Tl, Nb, moderate reactive, 3 valence electrons
14: Carbon group: C, Di, Ge, Sn, Pb, Fl. Can form covalent bonds, 4 valence electrons
15: Nitrogen group (Pnictogens): N, P, As, Sb, Bi, Mc, essential for life, 5 valence electrons
16: Oxygen group (Chalcogens): O, S, Se, Te, Po, Lv 6 valence electrons
17: Halogens: F, Cl, Br, I, At, Ts, very reactive, 7 valence electrons
18: Noble gases: He, Ne, Ar, Kr, Xe, Rn, Og, not reactive, full valence shell
Elements in the same group have the same number of valence electrons, which determines their bonding behavior.
For instance, the name halogens (translated: salt formers) comes from their tendency to form salts (eg. NaCl), especially with metals. Each halogen has 7 valence electrons, seeking one more according to the octet rule.
Transition metals (Groups 3-12)
Transition metals are found in the middle of the periodic table. Usually they have two valence electrons, but their d-orbitals lead to special bonding rules. They are good conductrots with low ionization energy.


©Copyright. All rights reserved.
Wir benötigen Ihre Zustimmung zum Laden der Übersetzungen
Wir nutzen einen Drittanbieter-Service, um den Inhalt der Website zu übersetzen, der möglicherweise Daten über Ihre Aktivitäten sammelt. Bitte überprüfen Sie die Details in der Datenschutzerklärung und akzeptieren Sie den Dienst, um die Übersetzungen zu sehen.