sp Hybridization
Atoms bind to form molecules, creating the structures that make you and everything around you. But how do they bond? To understand hybridization, you should be familiar with the concept of orbitals. Click here to read the article about orbitals.
Atoms bond by overlapping atomic orbitals, forming molecular orbitals that describe the electron density and distribution within a molecule.
Sigma and Pi Bonds
Covalent bonds are classified based on how orbitals overlap:
- Sigma bonds form by direct overlap of orbitals along their internuclear axis (straight line connecting the nuclei of two bonded atoms)- like s-s overlaps. Sigma bonds allow free rotation around the bond axis.
- Pi bonds form by the sideways overlap of p orbitals, creating electron density above and below the internuclear axis. P bonds restrict rotation and occur in double and triple bonds alongside a sigma bond.
Examples of Bonding
- single bond (C-H) => one sigma bond
- double bond (O=O) => one sigma bond + one pi bond
- triple bond (N=-N) => one sigma bond + two pi bonds
Hybridization
Consider carbon:
- Carbon has tow electrons in the 1s orbital (lowest energy level).
- It has four electrons on the second energy level: two in the 2s orbital and one in two of the 2p orbitals (one 2p orbitals remains empty)
- Since covalent bonds only form when orbitals with only one electron overlap, carbon should only form two bonds. However, in reality, carbon forms four bonds.
- This is explained by hybridization, where orbitals mix and recombine to form new orbitals.
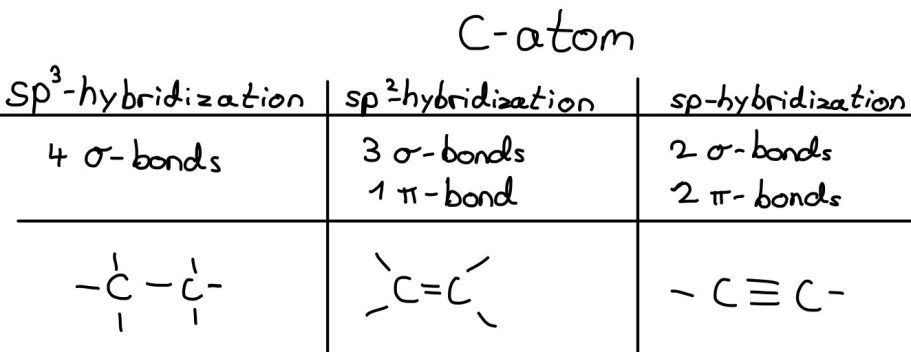
Sp^3 hybridization (single bonds)
Example: Methane (CH4)
The 2s orbitals and all three 2p orbitals of carbon mix and form four sp^3 hybidorbitals.
Each sp^3 orbital contains one electron, allowing four equivalent sigma bonds with hydrogen.
Hydrogen has one electron at the 1s orbital while carbon has one electron at each of the four sp^3 orbitals. They overlap and form bonds.
Conclusion: In sp^3 hybridization, three p orbitals and one s orbital mix, forming four sp^3 hybrid orbitals.
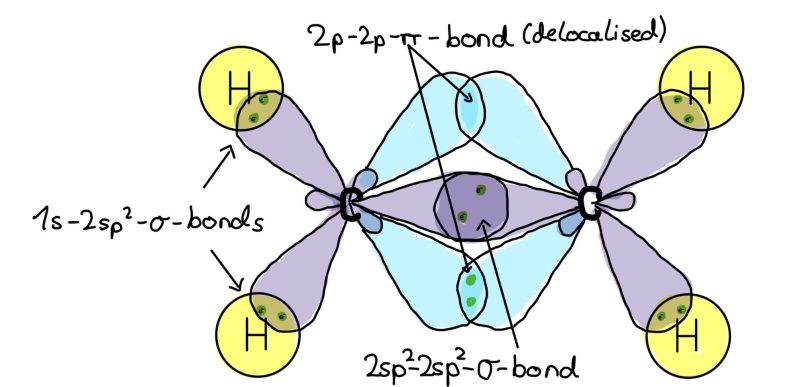
Sp^2 hybridizations (double bonds)
Example: Ethene (C2H4)
Sp^2 hybridization is found in double bonds, like between the carbon atoms in ethene.
The 2s orbital and two 2p orbitals of carbon mix and form three sp^2 hybrid orbitals. Those have a slightly higher energy level than the 2s orbitals and a slightly lower one than the 2p orbitals.
Each hybrid orbital has one electron, and the remaining unhybridized p orbital forms a pi bond through sideways overlap, creating the double bond.
In ethene, the sp^2 orbitals of carbon overlap with two hydrogen 1s orbitals and with the other carbon atom. The p orbitals of the carbon atoms overlap to form 2p-2p-pi-bonds.
Due to the shape of the 2p-2p-pi-bond, the location of the electrons is always uncertain, thus double bonds are delocalized.
Double bonds restrict the atoms to rotate freely and restrict their mobility.
Conclusion: in sp^2 hybridization, two p-orbitals and one s-orbitals mix, forming three sp^2 hybrid orbitals.
Sp hybridization (triple bonds)
Example: Ethyne (C2H2)
Sp hybridization is found in triple bonds, like the triple bond between the carbon atoms of ethyne.
The 2s and 2p orbital of carbon mix, forming two sp hybrid orbitals.
The remaining two unhybridized p orbitals form two pi bonds by sideways overlap. Together with the sigma bond with the sp hybrid orbitals, the create the triple bond.
Conclusion: in sp hybridization, one p-orbital and one s-orbital mix to two sp hybrid orbitals.
Hybridization explains atom-to-atom bonding and the most basic structures of our world. It is the process of mixing orbitals of the same energy level to produce hybrid orbitals that influence molecular geometry and bonding properties

©Copyright. All rights reserved.
Wir benötigen Ihre Zustimmung zum Laden der Übersetzungen
Wir nutzen einen Drittanbieter-Service, um den Inhalt der Website zu übersetzen, der möglicherweise Daten über Ihre Aktivitäten sammelt. Bitte überprüfen Sie die Details in der Datenschutzerklärung und akzeptieren Sie den Dienst, um die Übersetzungen zu sehen.